Background: Secondary CNS lymphomas (SCNSL) are aggressive and there are few treatment options. CNSLs often have underlying chronic active B-cell receptor signaling that responds to BTK inhibitors, but the response duration is short. TEDDI-R achieves durable remissions in primary DLBCL of the CNS (PCNSL), but the molecular profile of SCNSL tumors that are BTK-responsive is unknown. We present results from an ongoing response-adapted study of ibrutinib with TEDD-R in SCNSL [NCT03964090].
Methods: Pts with aggressive B-cell lymphomas with CNS +/- systemic involvement are eligible if age ≥18, PS<3, and adequate organ function. Pts are untreated with CNS involved or relapsed in the CNS after therapy. Prior BTKi is allowed. HIVis excluded. Baseline tests: brain MRI, PET/CT of body, bone marrow (BM), CSF flow cytometry, and fundoscopic exam. Pts receive isavuconazole as fungal prophylaxis throughout therapy. Pts first receive ibrutinib 560mg x 14d in a treatment window to determine ibrutinib-responsiveness. Pts with ≥20% reduction after ibrutinib receive TEDDI-R, and if <20% reduction receive TEDD-R (no ibrutinib). Chemotherapy is 4 cycles x21d as an outpatient and includes intrathecal chemotherapy. There is no maintenance therapy or planned consolidation. Response is assessed after cycles 2 and 4. Complete responses (CR) are confirmed with FDG-PET brain/body, CSF analysis, and BM. Surveillance scans are q3m for 1y, q4m x 1y, q6m x 1y, then annually. The primary endpoint is PFS.
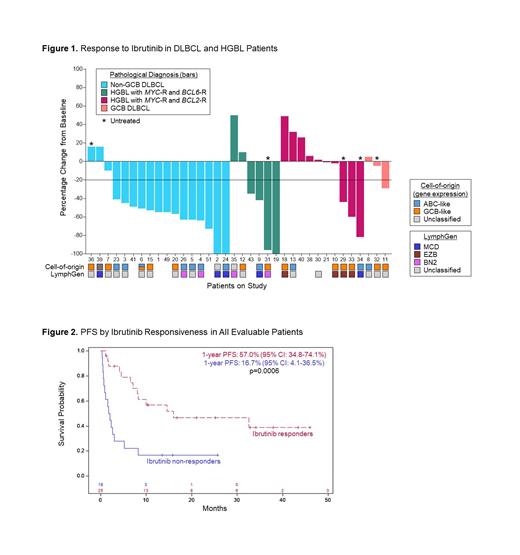
Results:50 pts enrolled between June 2019 and March 2023 with median age 63 (range 26-89), and 15 (30%) aged ≥70. 17 pts (34%) were female. 28 (56%) pts had DLBCL: 21 (42%) non-GCB, 5 (10%) GCB, 1 (2%) PMBL, and 1 (2%) unknown. 16 (32%) pts had HGBL: 10 (20%) with MYC/ BCL2-R and 6 (12%) with MYC/ BCL6-R. Two (4%) pts each had MCL and EBV-LPD, and 1 pt each had plasmablastic (2%) and Burkitt (2%). Five (10%) pts were treatment naïve and 45 (90%) were relapsed or refractory. 25 (50%) had synchronous CNS and peripheral disease, and 25 (50%) were isolated to the CNS. All relapsed or refractory pts had prior anthracycline, 29 (58%) prior HD-MTX, and 8 (18%) prior CAR-T. Forty-three pts completed the ibrutinib window and were evaluable for responsiveness; 25 (58%) were ibrutinib-responsive and 18 (42%) were ibrutinib-resistant (Figure 1). The overall response rate (ORR) to TEDDI-R (n=28) was 96%, including a CR rate of 71%. The overall response rate to TEDD-R (n=20) was 35%, with a CR rate of 25%. G3 or G4 neutropenia occurred in 43% and 31% of cycles, respectively, and febrile neutropenia occurred in 11%. G3 or G4 thrombocytopenia occurred in 31% and 12% of cycles, respectively, and G3 anemia occurred in 37%. G3 non-hematologic AEs (% pts) included UTI (20%), hypokalemia (22%), sepsis (14%), diarrhea (16%), hypotension (12%), fatigue (10%), anorexia (10%), adrenal insufficiency (10%), and atrial fibrillation (8%). Few G4 AEs were noted. No opportunistic infections (including Aspergillus) were observed. 14 (28%) pts had hand-foot syndrome.
After a median follow-up of 24m, the 1-year PFS and OS of all patients were 37.8% and 59.9%, respectively. Pt deaths were due to disease progression (20 pts, 83%), pneumonia (3 pts, 13%), and a cardiac event (1 pt, 4%). The 1-year PFS and OS for pts with ibrutinib-responsive compared to ibrutinib-resistant tumors were 57.0% vs. 16.7% (p=0.0006) and 73.8% vs. 47.9% (p=0.06) (Figure 2). Most (86%) ibrutinib-responsive DLBCL/HGBL tumors were CD10-negative. CD10-negative DLBCL/HGBL pts had an ORR of 77% and a CR rate of 70%. The 1-year PFS and OS for pts with CD10-negative DLBCL/HGBL were 50.5% and 65.0%, respectively. There was no difference in PFS or OS in pts aged <70 versus ≥70 (p=0.87 and p=0.74, respectively). We performed comprehensive genomic profiling on 27 DLBCL/HGBL tumors according to the LymphGen algorithm. 80% (n=4) MCD, 75% (n=3) BN2, 50% (n=2) EZB, and 50% (n=7) unclassified tumors were responsive to ibrutinib.
Conclusions: Pts with ibrutinib-responsive SCNSL achieve a high rate of CR to TEDDI-R, and preliminary data suggest these remissions are durable. Most ibrutinib-responsive tumors are CD10-negative and enriched for MCD and BN2. TEDDI-R can be delivered as an outpatient to pts of all ages.
OffLabel Disclosure:
Dunleavy:Genentech: Research Funding. Lai:Jazz: Consultancy, Research Funding, Speakers Bureau; BMS: Consultancy; Novartis: Consultancy; Rigel: Consultancy; Daiichi: Consultancy; Taiho: Consultancy; Pfizer: Consultancy; Genentech: Consultancy; Astellas: Consultancy, Speakers Bureau; AbbVie: Consultancy. Ibrahimi:Ipsen Bio, BMS, Sobi Inc: Consultancy.
Ibrutinib. FDA approved for treatment of hematologic malignancies and graft versus host disease.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal